The idea that a single infusion of a patient’s own immune cells could clear aggressive cancer once seemed like daring fiction. CAR T‑cell therapy has turned that notion into clinical fact, standing shoulder to shoulder with surgery, radiotherapy and chemotherapy as a fourth cornerstone of oncology. Unlike a chemical drug, the treatment is a living medicine: T‑cells are harvested, genetically re‑equipped with a synthetic chimeric antigen receptor, multiplied, then returned to the bloodstream to hunt malignant cells with unerring accuracy.
Fun fact: In 2012 one of the first adults to receive experimental CAR T‑cells at the University of Pennsylvania saw her refractory leukaemia vanish within three weeks and has remained cancer‑free ever since, making her one of the earliest long‑term survivors of the therapy.
From Coley’s toxins to gene‑edited killers
Cancer immunotherapy is not new. In the 1890s, surgeon William Coley injected heat-killed bacteria into tumours and observed some of them melt away, although the mechanisms were obscure. The modern era began in 1987 when Yoshikazu Kurosawa sketched the first chimeric T‑cell receptor. Two years later, Zelig Eshhar built “T‑bodies”, fusing antibody fragments to T‑cell signalling domains. Early constructs failed in patients because they lacked the survival cues that keep lymphocytes active in vivo.
The decisive step arrived in 1998 when Michel Sadelain added a co-stimulatory CD28 module, creating second-generation receptors that could expand, persist, and kill. A 2010 Philadelphia trial led by Carl June then produced dramatic responses in chronic lymphocytic leukaemia. In 2017, Novartis’s Kymriah became the first CAR T product licensed by the US Food and Drug Administration.
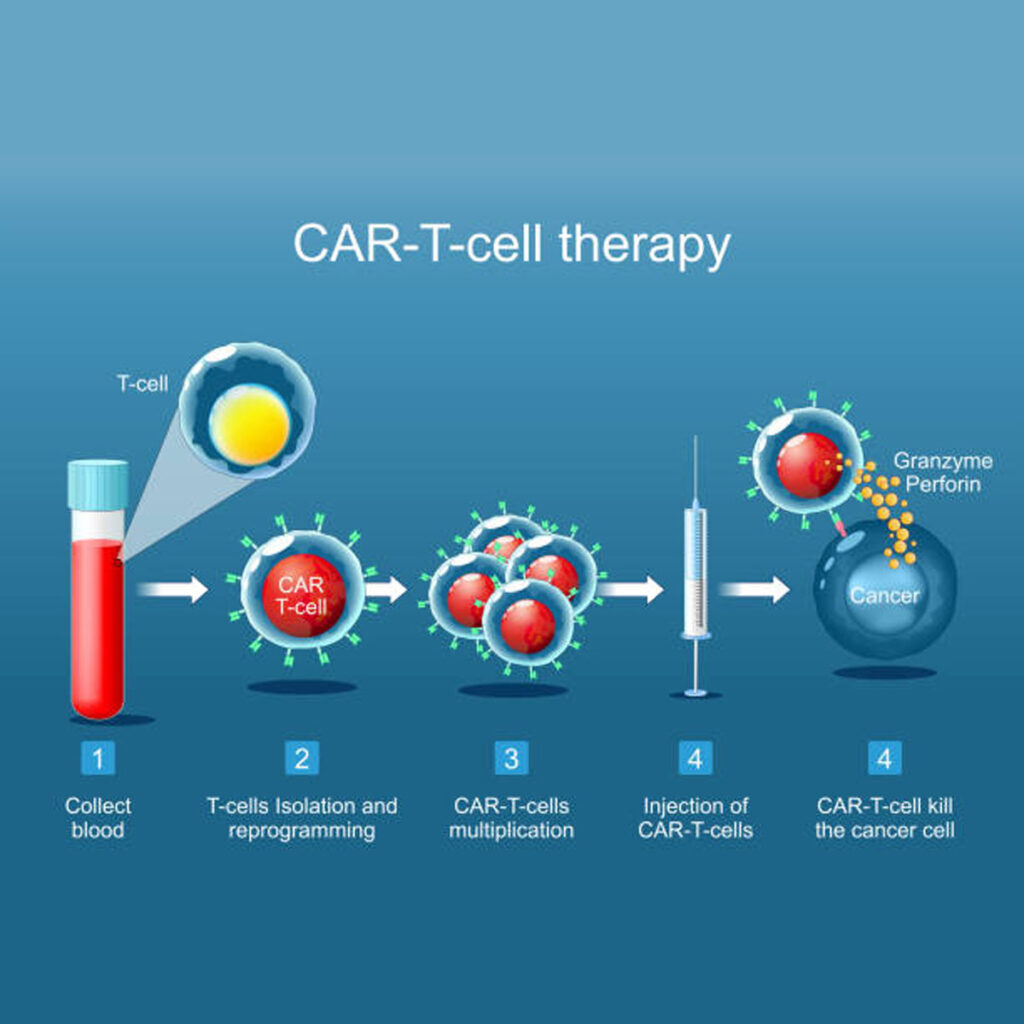
The vein‑to‑vein journey
- Leukapheresis – blood is filtered to collect T‑cells, a four‑hour outpatient procedure.
- Genetic engineering – a disabled lentivirus inserts DNA coding for the new receptor.
- Ex‑vivo expansion – modified cells grow to hundreds of millions over roughly 20 days.
- Lymphodepleting chemotherapy – fludarabine plus cyclophosphamide clears space for the incoming army.
- Infusion – thawed CAR T‑cells run into a peripheral vein in less than an hour.
- In‑vivo action – the cells proliferate, release perforin and granzymes, and form memory subsets for long‑term surveillance.
Manufacturing typically takes four to six weeks. During this window, clinicians may deliver bridging chemotherapy to hold the disease in check, a balancing act that can add extra toxicity.
Dissecting the synthetic receptor
- Extracellular scFv – derived from a monoclonal antibody, binds the tumour antigen without needing major histocompatibility complex display.
- Hinge or spacer – sets the distance betweenthe T‑cell and target, influencing synapse quality.
- Transmembrane anchor – fixes the receptor in the plasma membrane.
- CD3ζ activation motif – fires the primary kill signal.
- Co‑stimulatory domain – CD28 gives fast, forceful responses, whereas 4‑1BB favours steady endurance. All licensed products use one or the other.
Generations of CAR design
- First‑generation – CD3ζ only, inadequate persistence.
- Second‑generation – CD3ζ plus one co‑stimulator, now the clinical standard.
- Third‑generation – two co‑stimulators combined; benefits unproven, risk of tonic signalling.
- Fourth‑generation (TRUCKs) – secrete cytokines such as IL‑12 to inflame the tumour micro‑environment.
- Fifth‑generation – integrate cytokine receptor motifs for physiological proliferation cues and include logic‑gate safety switches.
Licensed treatments in the UK and EU
| Trade name | Generic | Target | Co‑stimulator | Core indications * |
| Kymriah | Tisagenlecleucel | CD19 | 4‑1BB | Paediatric/young‑adult relapsed or refractory (r/r) B‑ALL; adult r/r DLBCL; adult r/r follicular lymphoma |
| Yescarta | Axicabtagene ciloleucel | CD19 | CD28 | Adult r/r DLBCL or high‑grade B‑cell lymphoma; adult r/r PMBCL; adult r/r follicular lymphoma; second‑line in high‑risk LBCL |
| Tecartus | Brexucabtagene autoleucel | CD19 | CD28 | Adult r/r mantle‑cell lymphoma; adult r/r B‑ALL (≥ 26 years) |
| Breyanzi | Lisocabtagene maraleucel | CD19 | 4‑1BB | Adult r/r DLBCL, HGBL, PMBCL, follicular lymphoma |
| Abecma | Idecabtagene vicleucel | BCMA | 4‑1BB | Adult r/r multiple myeloma |
| Carvykti | Ciltacabtagene autoleucel | BCMA | 4‑1BB | Adult r/r multiple myeloma |
| Aucatzyl | Obecabtagene autoleucel | CD19 | 4‑1BB | Adult r/r B‑ALL (MHRA authorised) |
*Key National Institute for Health and Care Excellence (NICE) or European Medicines Agency (EMA) approvals current to early 2025.
Landmark trials that changed practice
- ELIANA – 81 % overall response in paediatric B‑ALL, 66 % two‑year overall survival.
- ZUMA‑7 – Yescarta as second‑line for large B‑cell lymphoma doubled event‑free survival versus transplant.
- CARTITUDE‑4 – Carvykti cut the risk of progression or death by 74 % in early‑relapse myeloma.
These data prompted the shift to earlier-line use, moving CAR T from salvage to potentially curative intent.

Toxicity: the price of potent immunity
Cytokine release syndrome
Fever, hypotension and hypoxia stem from interleukin‑6‑driven inflammation. Tocilizumab, an IL‑6 receptor blocker, plus supportive care reverses most episodes; high‑dose corticosteroids cover refractory cases.
Immune effector cell‑associated neurotoxicity
Confusion, aphasia and seizures may follow or accompany CRS. Neuro‑monitoring with the ICE score detects early change. Corticosteroids remain first‑line; tocilizumab is ineffective here.
Additional risks
- B‑cell aplasia – inevitable in CD19 therapies; lifelong immunoglobulin replacement often needed.
- Prolonged cytopenias – labelled ICAHT, can last months, raising infection and bleeding risk.
- Secondary T‑cell malignancies – extremely rare but under FDA and EMA review; every patient enters a 15‑year follow‑up registry.
Economics and access in Britain
The list price exceeds £280 000 per infusion, easily making CAR T one of the costliest interventions the NHS purchases. Confidential discounts and outcome‑based managed entry agreements soften the impact. A three‑layer governance model then decides access:
- Medicines and Healthcare products Regulatory Agency grants a licence.
- NICE judges cost-effectiveness, often through the Cancer Drugs Fund, to gather more evidence.
- National CAR‑T Clinical Panel vets each referral to allocate limited slots in the 16 accredited adult centres.
Patients must stay within an hour of the treating hospital for 30 days and provide a round-the-clock caregiver, which can exclude those without strong social or financial support.
A fast‑moving global marketplace
Market analysts project the CAR T‑cell therapy sector could climb from roughly USD 6 billion today to anywhere between USD 16 billion and USD 128 billion by the early 2030s, a reflection of expansion into solid tumours and earlier treatment lines. Yescarta currently leads revenue, but BCMA products are growing fastest. North America dominates sales, while China hosts the largest pipeline of clinical trials thanks to heavy government backing.
How regulators are adapting
- US FDA – first approvals, mandatory Risk Evaluation and Mitigation Strategies, active inquiry into secondary T‑cell malignancies.
- EMA – central authorisation plus continent‑wide safety registries, mirroring the FDA’s long‑term stance.
- UK MHRA – post‑Brexit independent body using Innovative Licensing and Access Pathway to accelerate promising cell therapies.
Coordinated pharmacovigilance treats these products more like transplants than pills, embedding decades‑long patient monitoring as standard practice.
Towards an off‑the‑shelf future
Autologous manufacture limits speed and cost. Allogeneic CAR T aims to bank healthy donor cells, edit out their T‑cell receptors to avoid graft‑versus‑host disease, and delete human leukocyte antigens to evade rejection. Gene-editing platforms, such as CRISPR and TALENs, lead the charge, while induced pluripotent stem cells could one day supply industrial-scale batches. The first allogeneic trials report encouraging safety, though achieving durable persistence remains the key hurdle.
Can solid tumours be cracked?
Haematological cancers present antigens on circulating cells; solid tumours cloak themselves behind dense stroma and immunosuppressive cytokines. Researchers are counterattacking with:
- Armoured CARs secreting IL‑12 to inflame the micro‑environment.
- Checkpoint‑blockade combinations to stop PD-1-mediated exhaustion.
- Dual-target and logic-gated designs that activate only when two antigens are present, thereby reducing healthy-tissue toxicity.
- Locoregional delivery, such as intraventricular infusions in glioblastoma, to bypass the blood-brain barrier.
Early‑phase results: a Claudin18.2 CAR achieved 38.8 % response in gastric and pancreatic cancer, while a B7‑H3 CAR for paediatric sarcoma is recruiting after promising pre‑clinical work.
Beyond oncology
The same precision that erases malignant B‑cells can reset dysregulated immunity. Small trials show CD19‑directed CAR T inducing drug‑free remission in systemic lupus erythematosus and refractory myasthenia gravis. HIV cure research is exploring CARs that target virally infected T‑cells hidden in reservoirs, opening a path to functional eradication of chronic infection.
Voices from the frontline
At the 2024 American Society of Haematology congress, clinicians argued that using CAR-T as a second-line treatment in aggressive lymphoma should become the norm. Survivorship specialists, meanwhile, called for structured mental‑health screening after treatment, noting emerging data on cognitive fatigue linked to prolonged cytokine exposure. Laboratory speakers highlighted T‑cell metabolic health as a predictor of response, advocating exercise and nutritional support during collection to harvest fitter lymphocytes.
Key debates that shape daily practice
- Product choice: CD28‑based CARs offer rapid tumour clearance but higher acute toxicity; 4‑1BB constructs expand more slowly yet endure longer. Tailoring the platform to tumour burden and patient fitness is becoming a refined art.
- Managing relapse: Antigen escape drives recurrence. Dual‑target CARs such as CD19/CD22 or sequential bispecific antibodies are being trialled to recapture resistant disease.
- Equity of access: Ultra-high prices, centralised centres, and caregiver needs disadvantage rural and lower-income patients. Advocacy groups urge decentralised manufacturing and travel grants to close the gap.
Where next?
Scientists see the current generation of CAR T as version 1.0. Success will be judged by three parallel advances:
- Smarter engineering – logic‑gated receptors that fire only in the exact biochemical postcode of a tumour, boosting safety.
- Manufacturing innovation – point‑of‑care platforms that complete engineering within 48 hours, slashing costs and wait times.
- Broadening indications – solid tumours, autoimmune disease and chronic infection stand to gain as lessons migrate across disciplines.
Conclusion: a living technology still evolving
CAR T‑cell therapy has redrawn the oncology landscape, converting once‑fatal blood cancers into curable conditions for many. Yet, like any revolution, its work is unfinished. The next breakthroughs will depend on collaborative science, equitable policy and relentless refinement. As one veteran haematologist told this reporter, “We have taught T‑cells new tricks; now we must teach health systems how to share them.” In other words, the race is no longer to build a better sword, but to ensure every soldier who needs one can wield it. When that goal is met, CAR T will stand as proof that precision biology can tame even the most complex diseases.








